SOLVED: Complete combustion of acetone is given by: C3H6O(l) + 4O2 –> 3CO2(g) + 3H2O(l). ΔH for this reaction is -1790 kJ. ΔHfo for O2 is 0 kJ, for CO2(g) is -393.5

The volume of `CO_2` prodcued by the combination of 40 ml of gaseous acetone in excess of oxygen is - YouTube

Calculate the volume of CO2 produced by the combustion of 40 mL of acetone in the presence of excess of oxygen.

Instantaneous acetone-PLIF images for the combustion case recorded at a... | Download Scientific Diagram

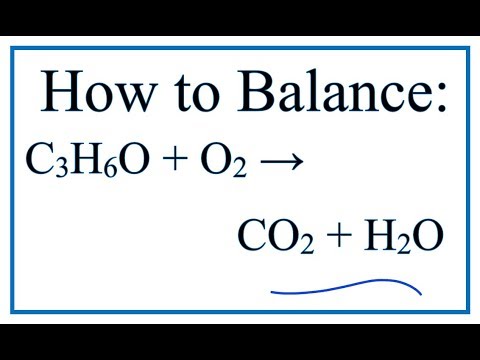
C3H6O+O2=CO2+H2O Balance the chemical equation. c3h6o+o2=co2+h2o acetone and oxygen reaction - YouTube
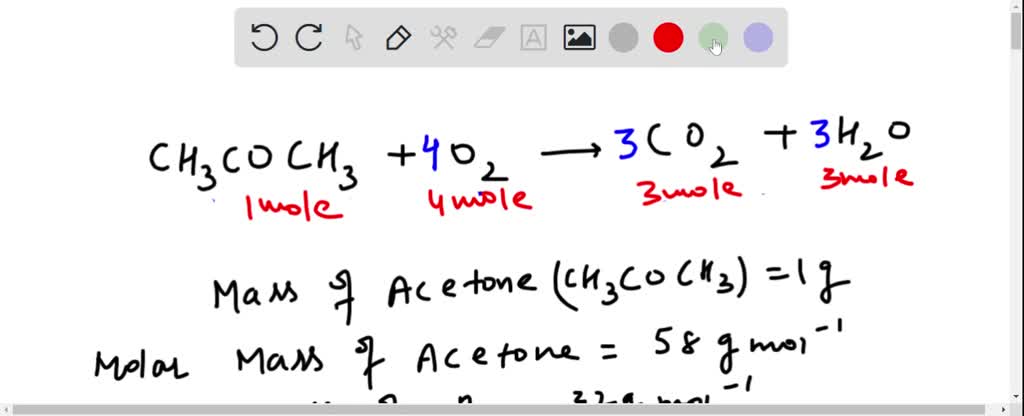
SOLVED: Write a balanced chemical equation for the combustion of acetone (CH3COCH3) in the presence of pure oxygen. Combustion products are carbon dioxide and water. What mass of oxygen is needed to
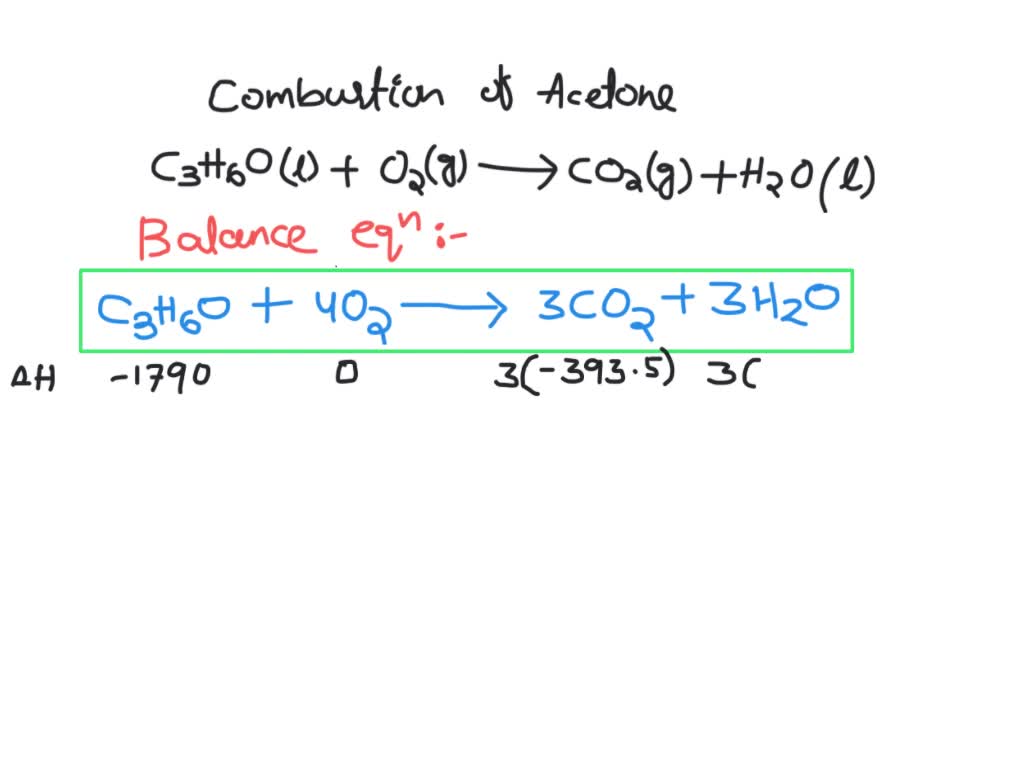
SOLVED: 5.4 Complete combustion of 1.00 mol of acetone (C3H6O) liberates 1790 kJ: C3H6O(l) + O2(g) â†' CO2(g) + H2O(l) (unbalanced) 5.4.1 Write the balanced thermochemical equation for the reaction. 5.4.2 Use










![ANSWERED] 59. Consider the thermochemical equation... - Inorganic Chemistry - Kunduz ANSWERED] 59. Consider the thermochemical equation... - Inorganic Chemistry - Kunduz](https://media.kunduz.com/media/sug-question/raw/57857865-1659726469.634809.jpeg)