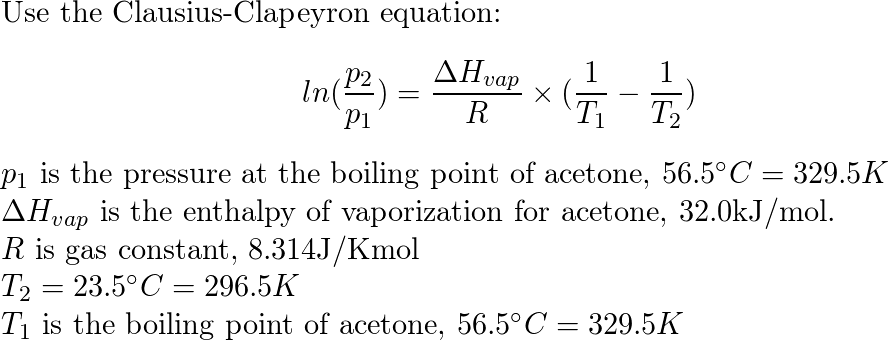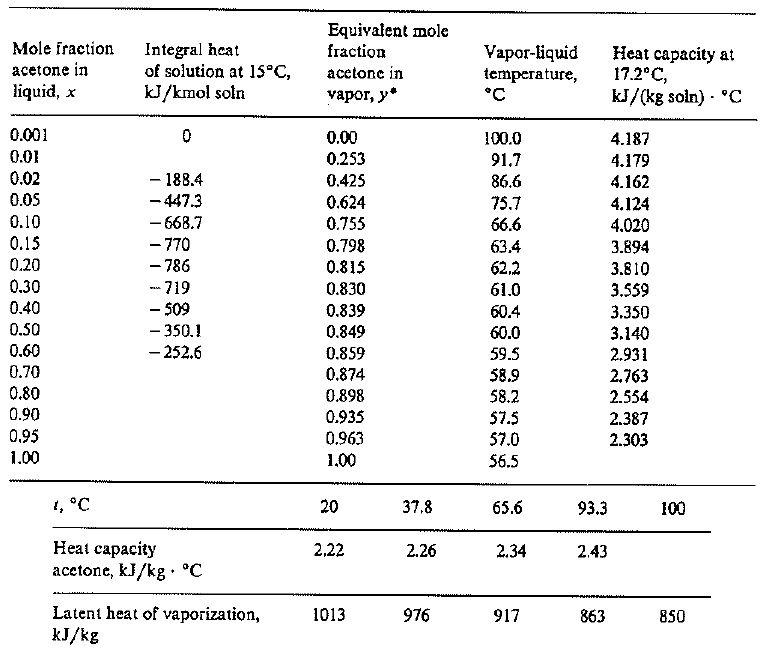
SOLVED: A liquid mixture containing 60 mol% acetone, 40 mol% water, at 26.7°C (80°F), is to be continuously flash-vaporized at 1 std atm pressure, to vaporize 30 mol% of the feed. (a)

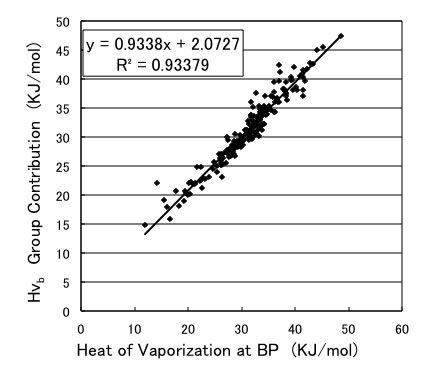
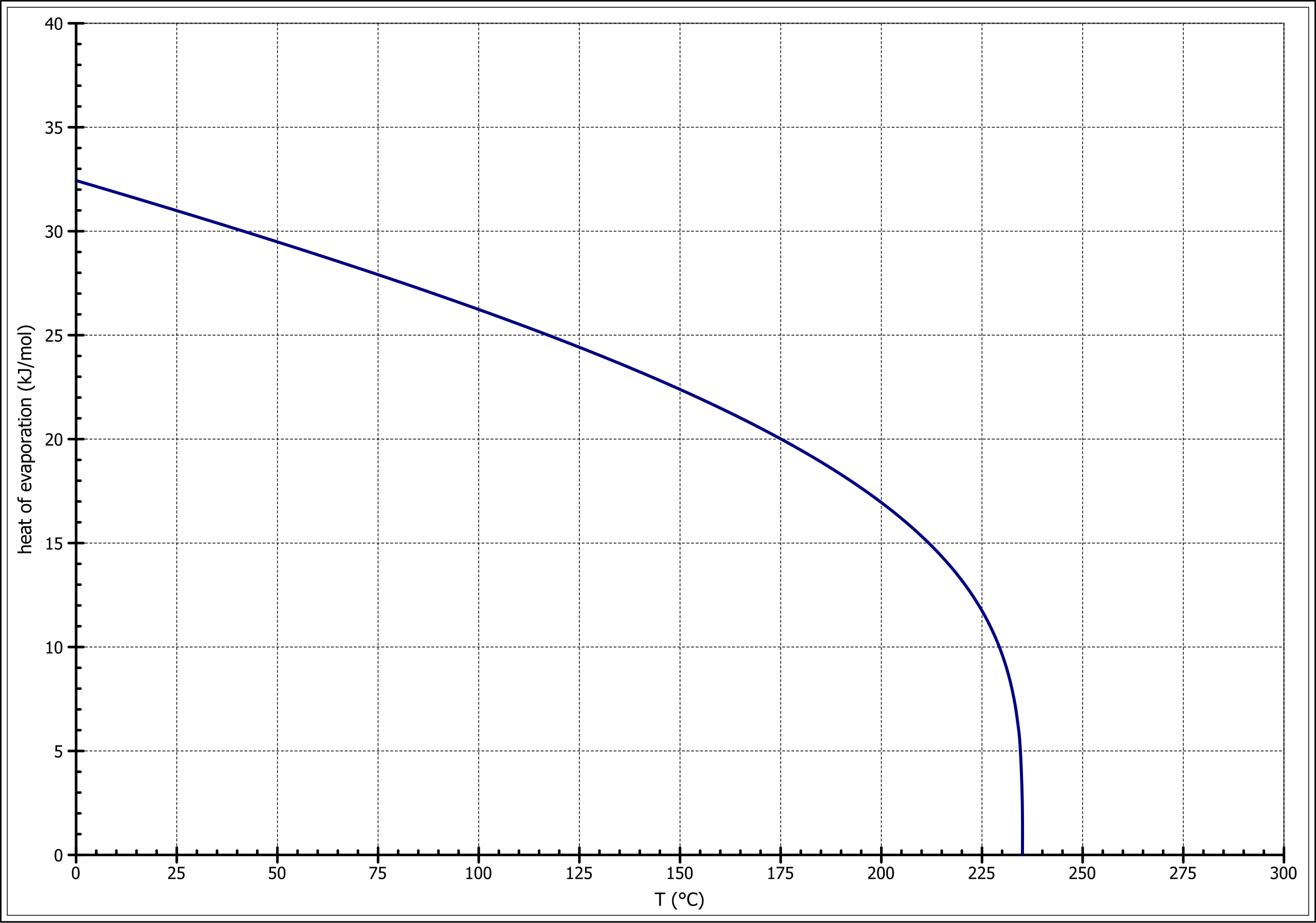
Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram

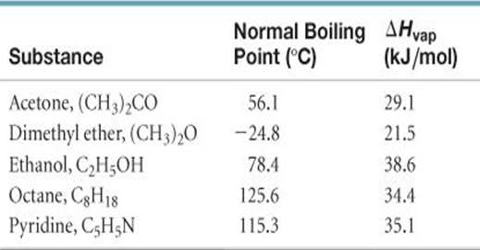
The enthalpy of vaporization for acetone is 320 kJ/mol The normal boiling point for acetone is 56 - YouTube
SOLVED: The vapor pressure of acetone (CH3COCH3) at 25°C is 228 torr. If the boiling point of acetone at 1.00 atm is 56°C, calculate the enthalpy of vaporization (ΔHvap; in kJ/mol) of

SOLVED:The enthalpy of vaporization for acetone is 32.0 kJ / mol . The normal boiling point for acetone is 56.5^∘ C . What is the vapor pressure of acetone at 23.5^∘ C ?

consider the molecular structures below. which of the following statements is correct? select all that - brainly.com

Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram

Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram
How to calculate the vapor pressure of acetone at 25.0°C if the enthalpy of vaporization for acetone is 32.0 kJ/mol and the normal boiling point of acetone is 56.5°C - Quora
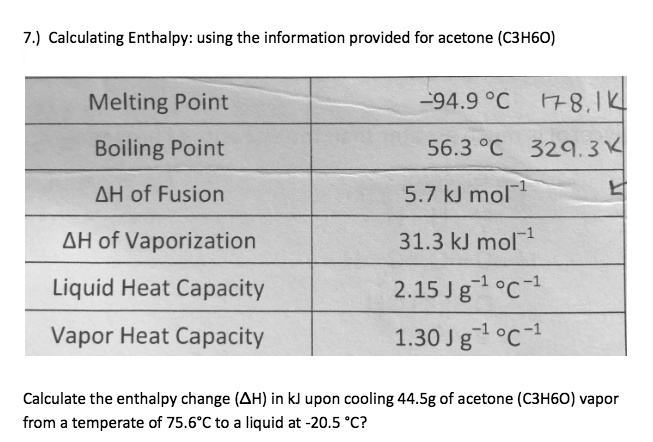
SOLVED: Calculating Enthalpy: using the information provided for acetone (C3H6O) Melting Point Boiling Point AH of Fusion 94.9 °C 56.3 °C 329.34 kJ mol-1 AH of Vaporization 31.3 kJ mol-1 2.15 J