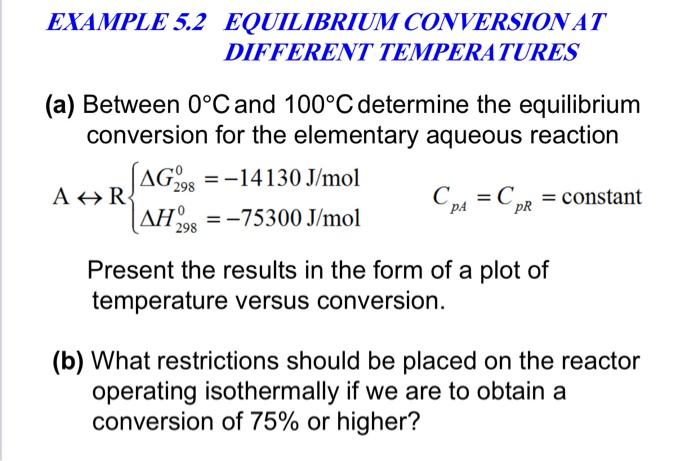
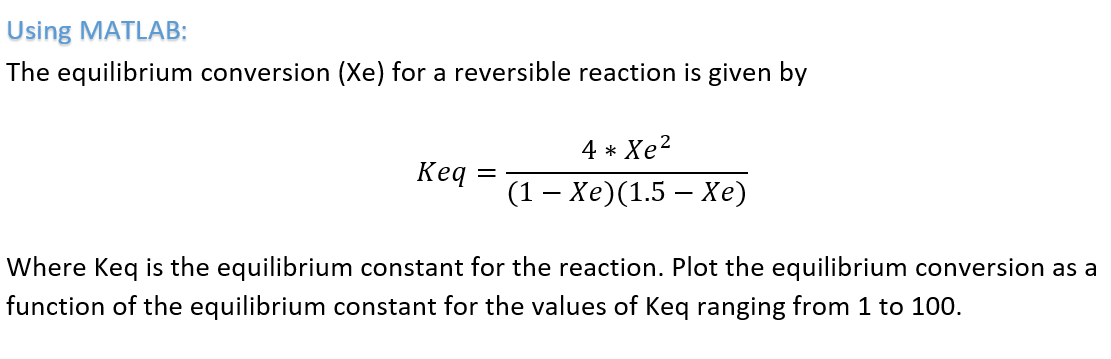A) Conversion B) Temperature C) Equilibrium constant and D) Reaction... | Download Scientific Diagram
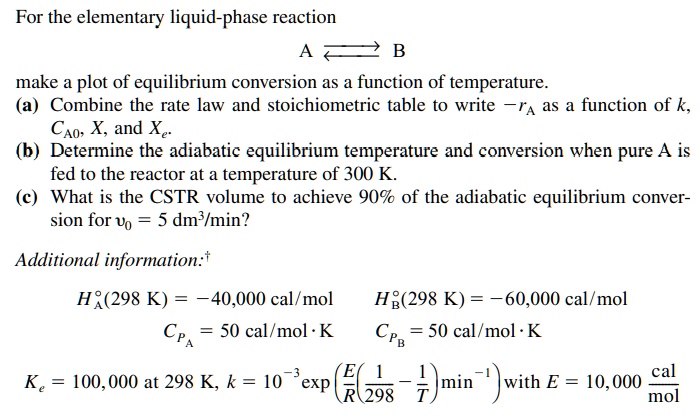
SOLVED: For adiabatic equilibrium temperature For the elementary liquid-phase reaction A + B -> C make a plot of equilibrium conversion as a function of temperature. (a) Combine the rate law and
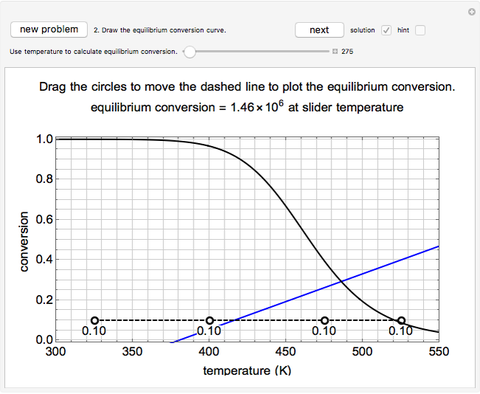
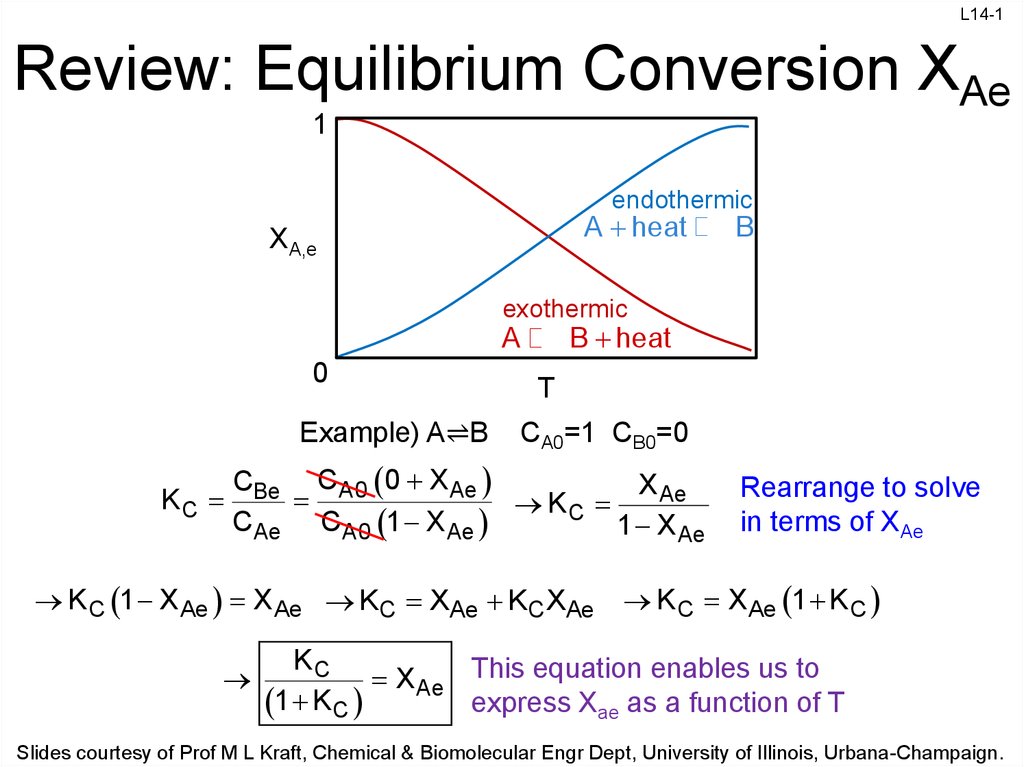
Construct a Conversion-Temperature Diagram for a Reversible Adiabatic Reaction - Wolfram Demonstrations Project

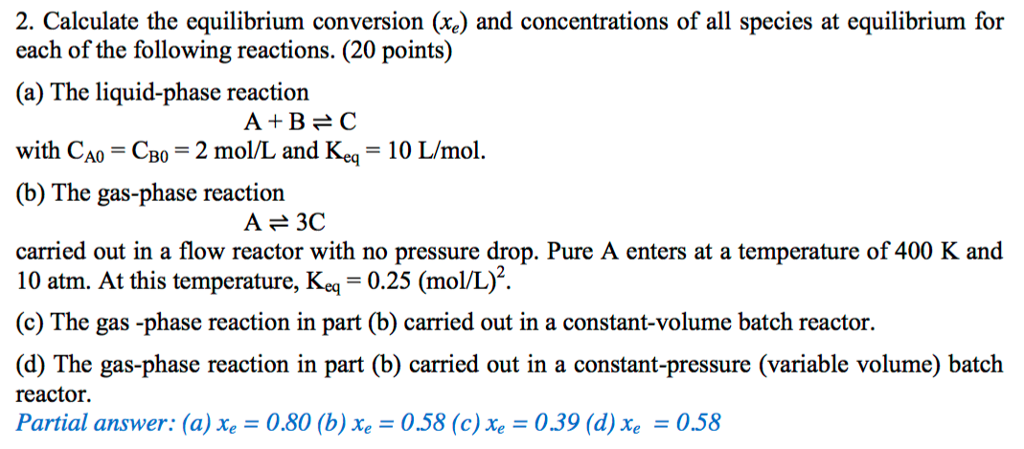
Equilibrium conversion when the pressure is 50 atm, Kp = 0.0016 by using Excel Goal Seek | TeacherOn

a) Equilibrium conversion of CO 2 and equilibrium selectivities of (b)... | Download Scientific Diagram